Additional information
| Active substance | Imatinib |
|---|---|
| Water Retention | Yes, can cause fluid retention |
| Hepatotoxicity | Low, but liver function tests are recommended |
| Lab Test | Monitoring of complete blood counts and liver function tests are advised |
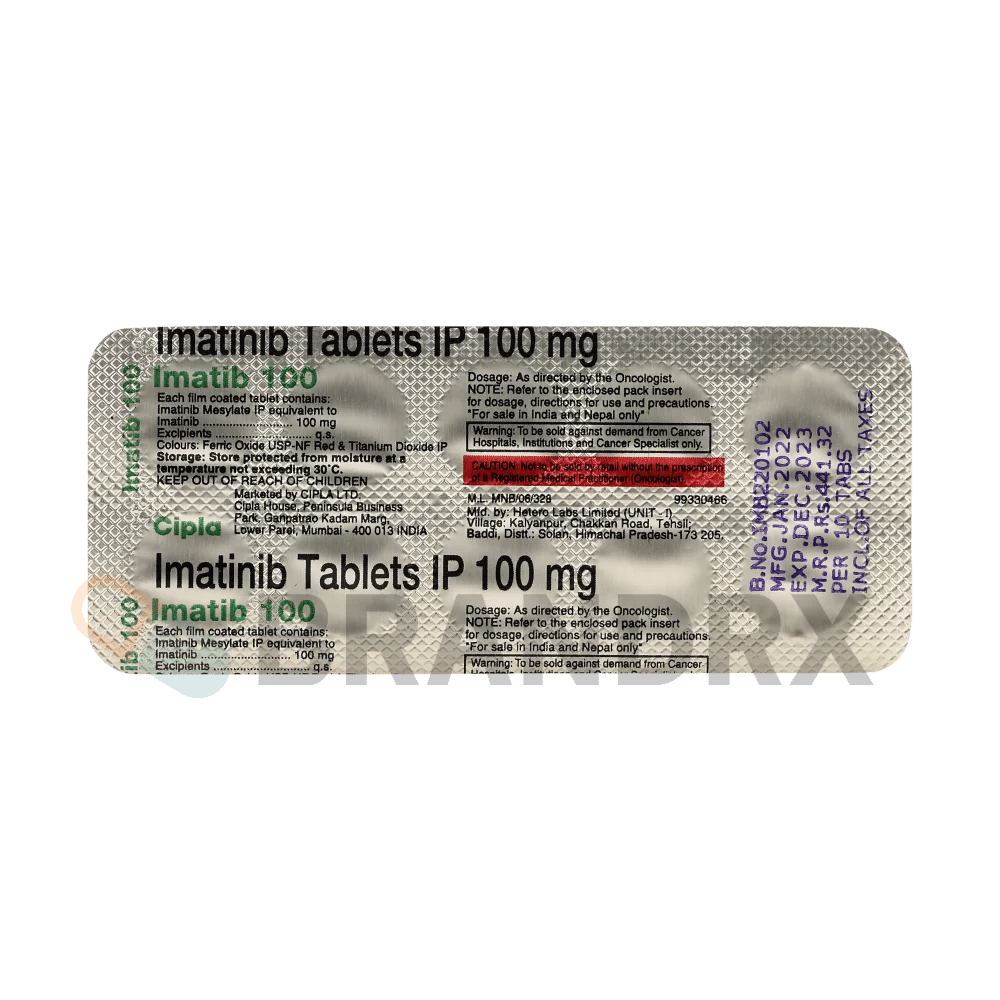
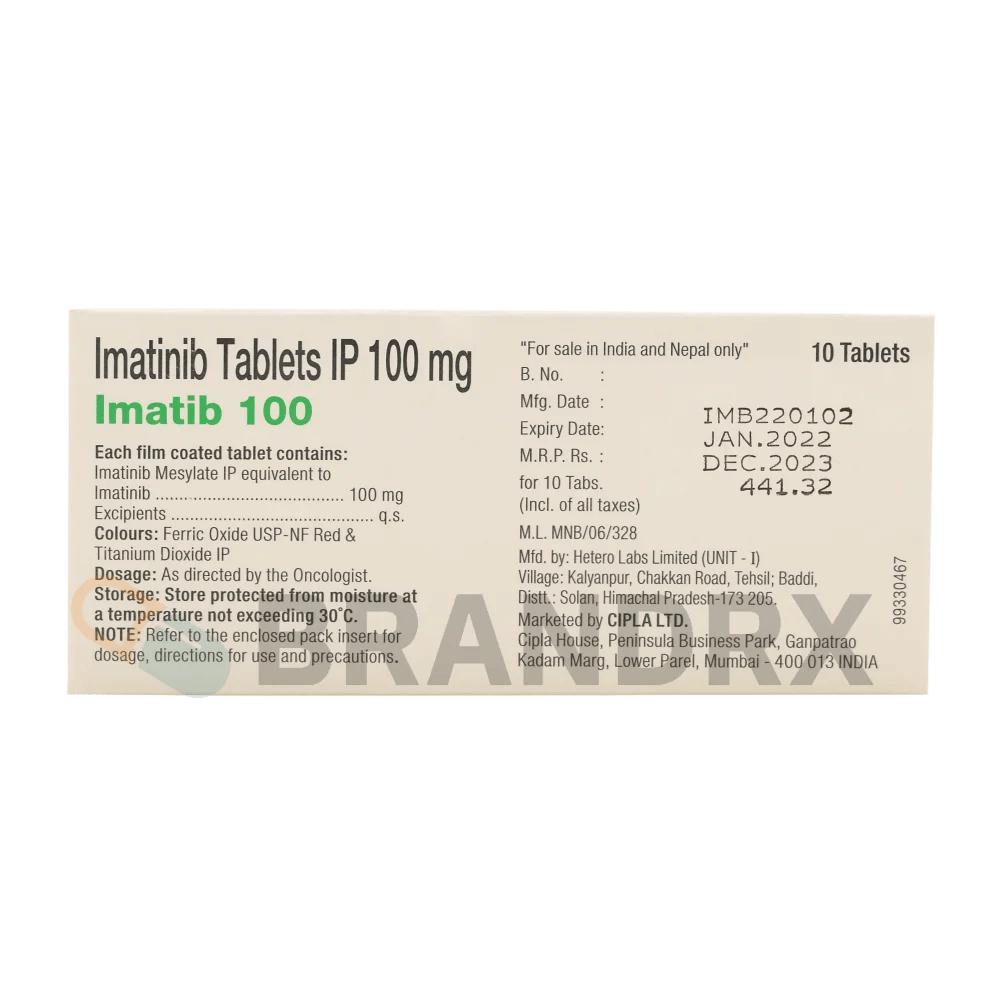
| Strength | 100mg |
| Also known as | STI571 |
| Blood pressure | Can cause changes in blood pressure, monitoring advised |
| Trade name | Gleevec (USA), Glivec (Europe and Australia) |
| Storage conditions | Store at room temperature away from moisture and heat |
| Chemical name | 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide |
| Formula | C29H31N7O |
| Substance class | Tyrosine kinase inhibitor |
| Main action | Inhibits the Bcr-Abl tyrosine kinase |
| Half-life | Approximately 18 hours |
| Dosage (medical) | Typically 400-800 mg per day, depending on the condition being treated |
| Dosage (sports) | Not applicable as it is not used for performance enhancement |
| Effects | Treats certain types of cancer by blocking the action of the abnormal protein that signals cancer cells to multiply |
| Side effects | Nausea, vomiting, edema, muscle cramps, musculoskeletal pain, diarrhea, rash, fatigue, abdominal pain |
| Use in sports | None, not used as a performance-enhancing drug |
| Manufacturer | Cipla Ltd. |
| Packing | 10 tabs/blister |




Reviews
There are no reviews yet.